Prof. Martin O. Saar, ETH Zurich
1 March 2016
DESTRESS: Horizon 2020 (European Commission) call:H2020-LCE-2015-2
Prof. Martin O. Saar, ETH Zurich
Description
This study is part of the DESTRESS project which follows the ambition to reliably increase system efficiency of enhanced geothermal systems (EGS). EGS uses artificial improvements of the hydraulic performance of a reservoir to allow a widespread usage of the huge untapped geothermal potential. The concept of chemical stimulation treatment of geothermal reservoirs has been developed by DESTRESS to optimize the EGS approach in a sustainable way and adapt it to the specific geological environments of Europe.
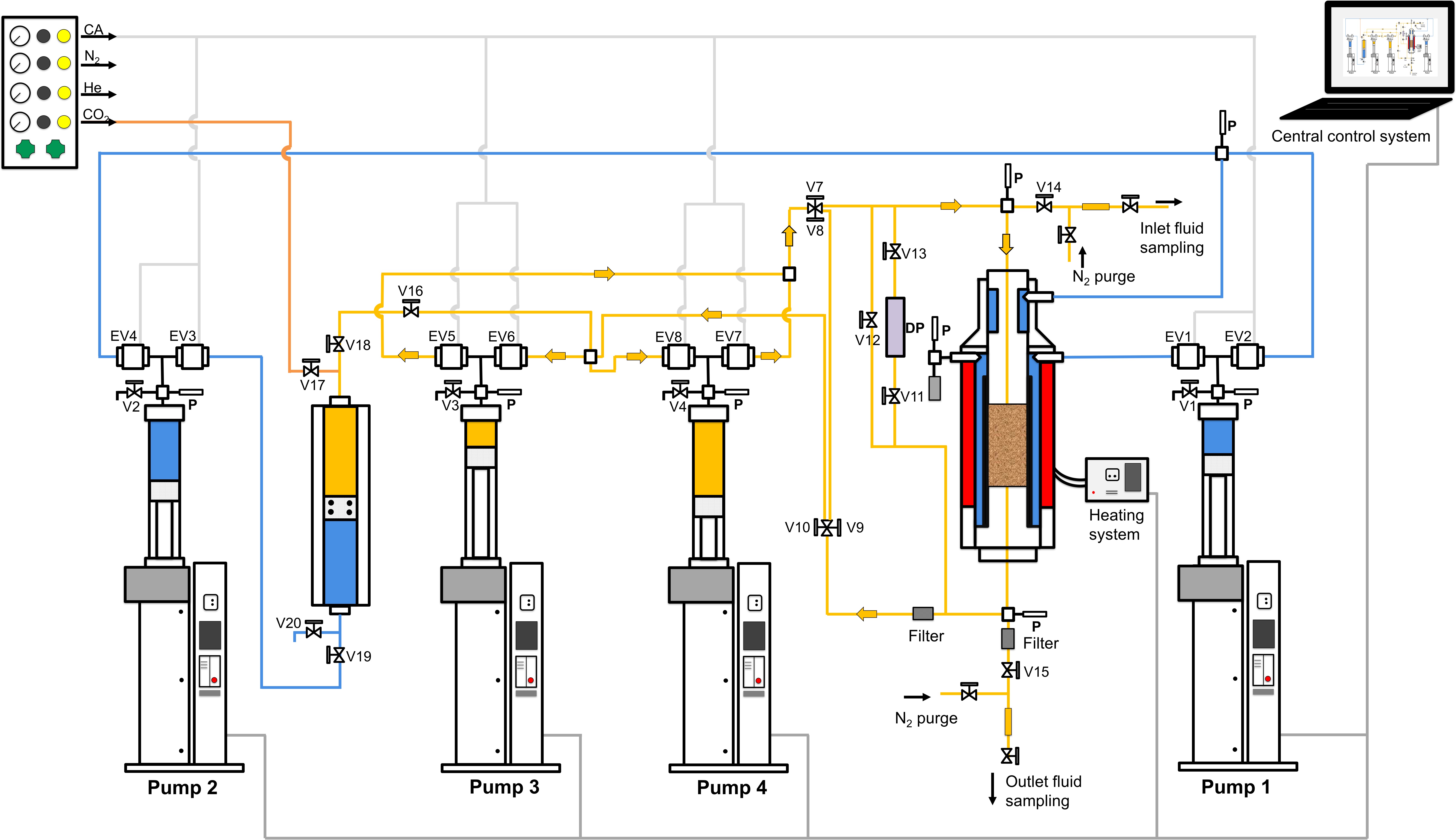
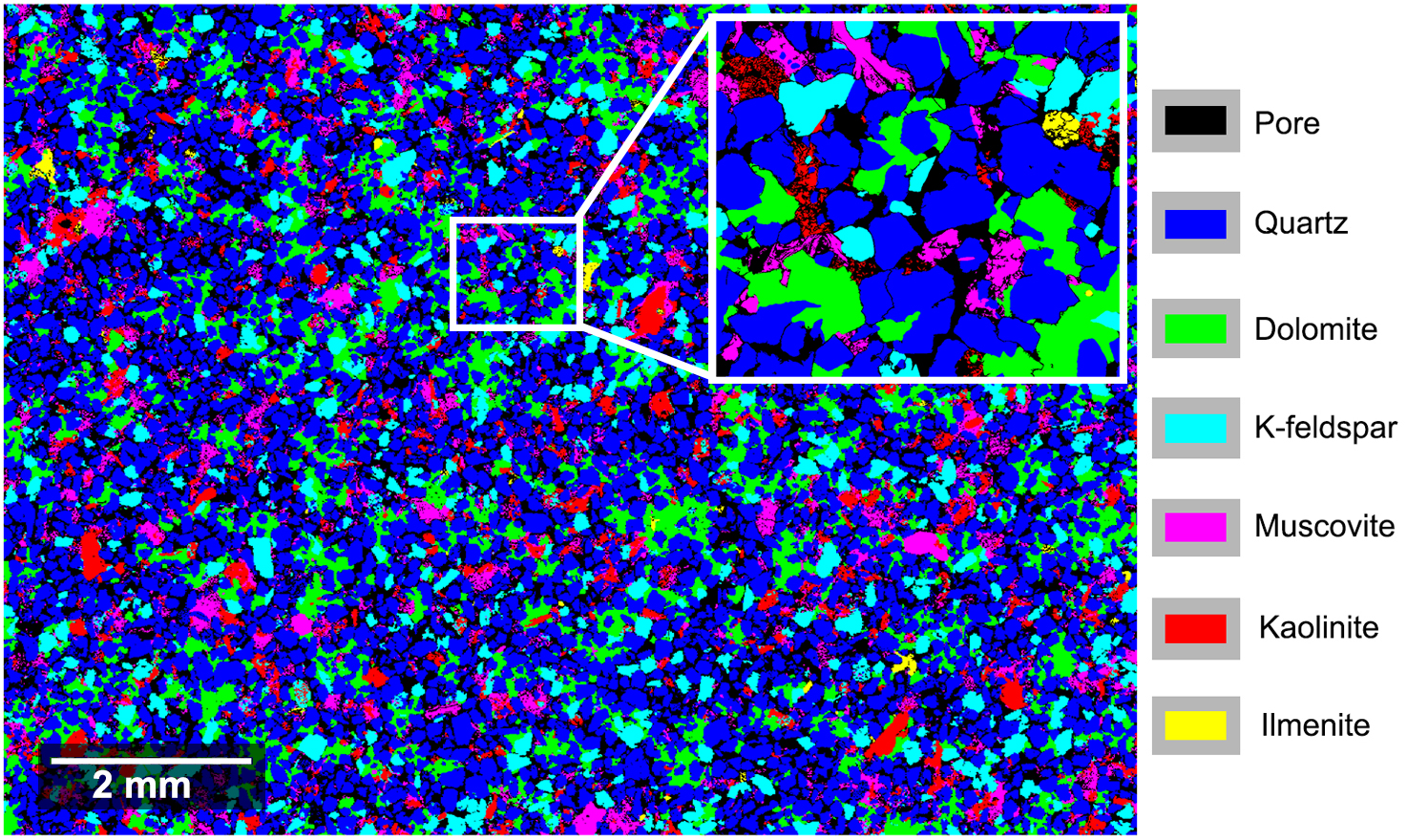
This study focuses on fluid-rock reactions and their consequences on rock properties of multi-mineral sandstones under geothermal reservoir conditions. An image-based approach is first developed to estimate the accessible surface area of each mineral using a Monte-Carlo algorithm where BET measurements are set as benchmark values of surface area. Then reactive flow-through experiments on sandstones are performed to investigate the reactivity of the reacting phase (dolomite) in a CO2-charged brine. The participation efficiency of the accessible surfaces is quantified to delineate the effective surface area (ESA) evolution during the experiments. The evolution of the efficiency and ESA are later confirmed independently using a 2D numerical dissolution model. Finally, this project investigates the effect of mineral dissolution on the effective stress law for the rock permeability. This project provides in-sights on the hydro-chemical-mechanical coupling processes in a multi-mineral system, with the applications on geological carbon sequestration and chemical stimulation of geothermal reservoir when CO2 is considered as the working fluid.
References
A.J. Luhmann and X.-Z. Kong and B.M. Tutolo and N. Garapati and B.C. Bagley and M.O. Saar and W.E. Seyfried Jr. Experimental dissolution of dolomite by CO2-charged brine at 100oC and 150 bar: Evolution of porosity, permeability, and reactive surface area, Chemical Geology, 380 pp.145-160 (2014)