Description
Introduction
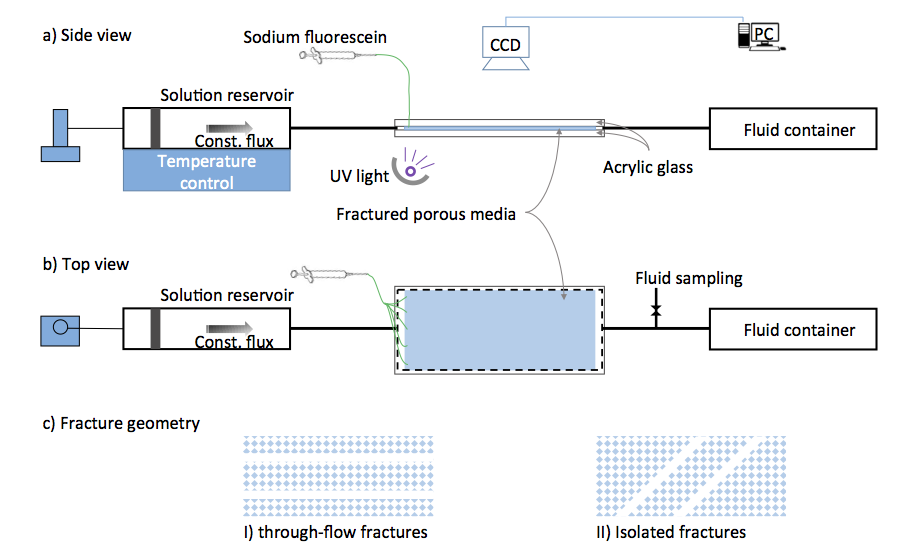 Sketch of the experimental setup. Slightly under-saturated solution with respect to calcite at controlled temperature in an upstream reservoir is pumped through a tube into a quasi-2D transparent reaction cell (a, b), where the calcite precipitates due to a temperature increase. Two types of analogue fractured porous media (c) will be installed in the reaction cell, where the porous medium is a single-layer etched acrylic glass.
Sketch of the experimental setup. Slightly under-saturated solution with respect to calcite at controlled temperature in an upstream reservoir is pumped through a tube into a quasi-2D transparent reaction cell (a, b), where the calcite precipitates due to a temperature increase. Two types of analogue fractured porous media (c) will be installed in the reaction cell, where the porous medium is a single-layer etched acrylic glass.
Utilization of underground reservoirs for geothermal energy extraction, CO2 storage, groundwater utilization, or waste fluid injection requires an in-depth understanding of fluid, solute (e.g., dissolved CO2, minerals, or waste fluids), and energy (heat, pressure) transport through often fractured porous geologic formations. Such operations necessarily perturb the chemical, thermal and/or pressure equilibrium between native fluids and rock minerals, potentially causing mineral dissolution and/or precipitation reactions with often immense consequences for fluid, solute, and energy transport, injectivity, and/or withdrawal in/from such reservoirs.
Mineral dissolution is typically less of a concern than mineral precipitation because mineral dissolution increases the ability of the fractured porous medium (reservoir) to inject, transmit, and extract fluids, solutes, and energy. Even though less studied, a much more significant problem is mineral precipitation, as, depending on the location of the mineral deposition, the ability of the reservoir to transmit fluids, solutes, and energy (i.e., the reservoir permeabillity) can reduce by many orders of magnitude. This potentially has detrimental consequences for geothermal energy usage, geologic CO2 storage, and waste fluid injection. Importantly, the amount of mineral precipitation that can cause orders of magnitude in permeability reduction can be very small, if minerals deposit in pore throats or narrow fracture apertures. However, understanding where mineral deposition occurs is non-trivial, as it depends on complex chemical, thermodynamic, and fluiddynamic feedback mechanisms.
The proposed study would investigate precisely the above-described mechanisms in laboratory experiments. Specifically, we will investigate the parameters that determine the depositional patterns of mineral precipitation in analogue fractured porous media, the mechanisms that govern the evolution of such mineral deposition, and the permeability changes that result from such pore-space modifications. By employing transparent acrylic glass as the front and back plates of an artificial fractured porous reaction cell, we can instantaneously register the depositional patterns of precipitated minerals using a calibrated camera. In addition, fluorescent dye tracing, illuminated by ultraviolet light, will visualize fluid streamlines within pores and fractures. Experimentally visualizing the flow field and simultaneously examining its role on mineral precipitation patterns is a novel approach. Three control parameters will be investigated: 1) the temperature difference between the solution reservoir and the reaction cell, 2) the cell inlet flow velocity, and 3) the fracture and the pore-space geometry. These experimental observations will serve as calibration examples for the development of a pore-scale fluid flow and fluid-mineral reaction simulator, a critical step towards simulating and understanding the longterm behavior of geologic reservoirs.
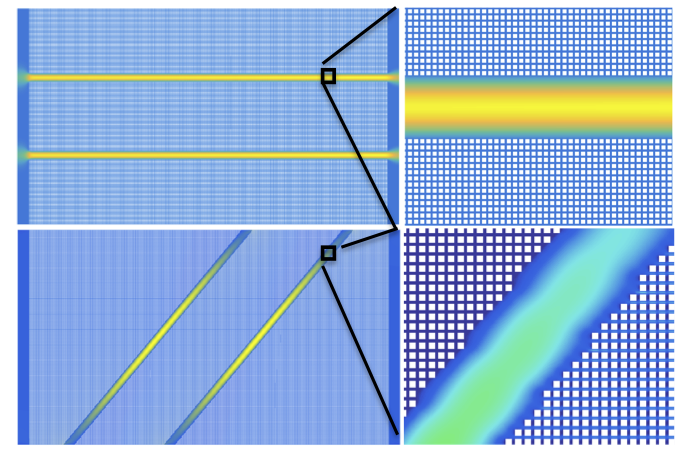 Fluid flow simulations under steady-state conditions showing fluid velocity fields for a flow-through fractured porous medium (top panel) and for an isolated-fracture porous medium (bottom panel) calculated using our lattice-Boltzmann numerical simulator, LBHydra. The same pressure gradient was maintained from the left to the right model boundary, together with no-flow conditions for the top and bottom boundaries. An enlarged portion of the fractured porous medium is also shown in each panel, where the white squares, with a size of 8×8 square lattices, are obstacles (the solid phase). A distance of 4 lattice nodes has been set between the obstacles. Two parallel fractures, with a distance of 960 lattice nodes, are placed in the porous medium. Each fracture has a width of 120 lattices nodes.
Fluid flow simulations under steady-state conditions showing fluid velocity fields for a flow-through fractured porous medium (top panel) and for an isolated-fracture porous medium (bottom panel) calculated using our lattice-Boltzmann numerical simulator, LBHydra. The same pressure gradient was maintained from the left to the right model boundary, together with no-flow conditions for the top and bottom boundaries. An enlarged portion of the fractured porous medium is also shown in each panel, where the white squares, with a size of 8×8 square lattices, are obstacles (the solid phase). A distance of 4 lattice nodes has been set between the obstacles. Two parallel fractures, with a distance of 960 lattice nodes, are placed in the porous medium. Each fracture has a width of 120 lattices nodes.
Experimental approach
The proposed project will investigate mineral deposition by making them optically visible in a 2D transparent reaction cell. Moreover, non-invasive techniques, such as fluorescent dye tracing, illuminated by UV light, will be applied to visualize the streamlines of pore fluid flow at a given sampling instance. This novel approach will allow simultaneous visualization and investigation of both the fluid flow field and associated fluid-mineral reactions and, in particular, mineral precipitation in fractures versus in surrounding pores and the effects of such precipitation on the bulk permeability and on the permeability field. While bulk permeabilties can be directly determined from the reaction cell experiment (by measuring overall flow rate, porefluid pressure gradient, and flow-perpendicular cell cross section), as it also serves as a standard Darcy permeameter, the permeability field can be determined from LBHydra lattice-Boltzmann (LB) fluid flow simulations. These simulations would be based on the above-mentioned streamline visualizations, as LB methods can serve as a numerical permeameters that can be applied to representative elementary volumes (REVs), or subsections, of the fractured porous medium. Taken together, the fluid flow velocity measurements, the geometric characterization of mineral precipitation, and the bulk permeability and permeability field determinations enable investigation and understanding of the complex interplay between fluid flow, precipitation kinetics, and the effects of evolving fracture and pore-space geometries.
References
Luhmann, A. J., X.-Z. Kong, B. M. Tutolo, K. Ding, M. O. Saar and W. E. Seyfried Jr (2012). Permeability reduction produced by grain reorganization and accumulation of exsolved CO2 during geologic carbon sequestration: A new CO2 trapping mechanism, Environmental science & technology, 47(1): 242-251.
Luhmann, A. J., X.-Z. Kong, B. M. Tutolo, N. Garapati, B. C. Bagley, M. O. Saar and W. E. Seyfried (2014). Experimental dissolution of dolomite by CO2-charged brine at 100 °C and 150 bar: Evolution of porosity, permeability, and reactive surface area, Chemical Geology, 380: 145-160.
Ma, Y., X. Z. Kong, A. Scheuermann, S. Galindo-Torres, D. Bringemeier and L. Li (2015). Microbubble transport in water‐saturated porous media, Water Resources Research, DOI: 10.1002/2014WR016019.
Randolph, J. B. and M. O., Saar (2011a). Coupling carbon dioxide sequestration with geothermal energy capture in naturally permeable, porous geologic formations: implications for CO2 sequestration. Energy Procedia, 4: 2206–2213.
Randolph, J.B. and M. O., Saar (2011b). Combining geothermal energy capture with geologic carbon dioxide sequestration. Geophys. Res. Lett., 38 (10): L10401. http://dx.doi.org/10.1029/2011GL047265.
Saar, M. O. and M. Manga (1999). Permeability‐porosity relationship in vesicular basalts, Geophysical Research Letters, 26(1): 111-114.
Saar, M. O. and M. Manga (2002). Continuum percolation for randomly oriented soft-core prisms, Physical Review E, 65(5): 056131.
Saar, M. O. and M. Manga (2004). Depth dependence of permeability in the Oregon Cascades inferred from hydrogeologic, thermal, seismic, and magmatic modeling constraints, Journal of Geophysical Research: Solid Earth (1978–2012), 109(B4).
Saar, M. O. (2011). Review: Geothermal heat as a tracer of large-scale groundwater flow and as a means to determine permeability fields, Hydrogeology Journal, 19(1): 31-52.
Tutolo, B. M., A. J. Luhmann, X.-Z. Kong, M. O. Saar and W. E. Seyfried Jr (2014). Experimental observation of permeability changes in dolomite at CO2 sequestration conditions, Environmental science & technology, 48(4): 2445-2452.
Tutolo, B. M., A. J. Luhmann, X.-Z. Kong, M. O. Saar and W. E. Seyfried (2015). CO 2 sequestration in feldspar-rich sandstone: Coupled evolution of fluid chemistry, mineral reaction rates, and hydrogeochemical properties, Geochimica et Cosmochimica Acta, 160: 132-154.
Walsh, S. D. and M. O. Saar (2008). Magma yield stress and permeability: Insights from multiphase percolation theory, Journal of Volcanology and Geothermal Research, 177(4): 1011-1019.
Walsh, S. and M. O. Saar (2010). Interpolated lattice Boltzmann boundary conditions for surface reaction kinetics, Physical Review E, 82(6): 066703.
Walsh, S. and M. O. Saar (2010b). Macroscale lattice‐Boltzmann methods for low Peclet number solute and heat transport in heterogeneous porous media, Water Resources Research, 46(7).